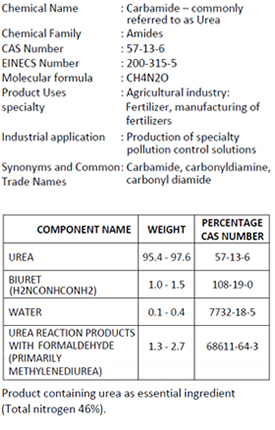
COMPOSITION / INFORMATION ON
INGREDIENTS
INGREDIENTS
READ THE ENTIRE LABEL BEFORE USING THIS PRODUCT. USE ONLY IN ACCORDANCE WITH INSTRUCTIONS. KEEP OUT OF REACH OF CHILDREN
|
What is Urea 46% (Carbamide) and how does it work?
Urea is a naturally occurring compound contained in urine from mammals. It is manufactured by combining carbon dioxide with ammonia and is the most commonly used nitrogen fertilizer worldwide. With more than 46% nitrogen, it has the highest nutrient concentration among the commercially available solid nitrogen fertilizers. It can be applied in a solid prilled or granulated form. Although soluble in water, its application in fluid form is uncommon. In the soil, urea is converted from carbamide nitrogen to ammonium ions (NH4 +) by a series of enzyme reactions. Under normal soil conditions, the ammonium ions are absorbed by the soil (i.e. become attached to the negatively charged soil particles) and the nitrogen becomes available to the plant, either in its ammonium form or as nitrate following microbial oxidation. Urea derived ammonium behaves in exactly the same way as that from other ammonium based nitrogen fertilizers. This breakdown of urea to release ammonium ions normally occurs within a week. The most favourable conditions for the efficient absorption of ammonium ions are:
Unfavourable conditions, such as:
converted from ammonium and released to the atmosphere (after the application of fertilizer). |



